Abstract
Introduction:
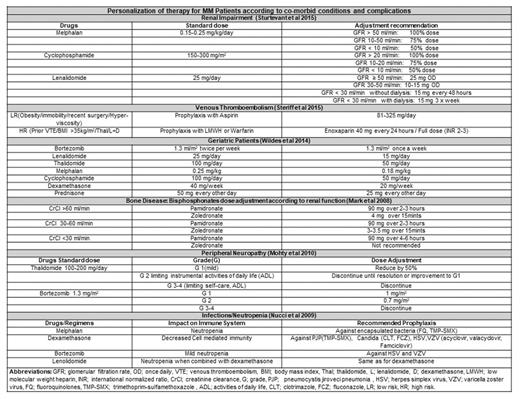
Personalization of Multiple Myeloma (MM) therapy according to individual patient requirements is an emerging need. Most patients with newly diagnosed MM are age 65 years or above and may have unique challenges due to comorbidities and risks for toxicity. Diverse prognostic factors, cytogenetic aberrations, age associated comorbidities, disease stage and systemic complications of MM call for tailoring the therapeutic regimens for MM according to each patient's unique presentation. Bone disease, renal impairment, venous thromboembolism, cytopenias, infections and neuropathy are the common complications of MM or its treatment. Novel agents and stem cell transplantation have improved the overall (OS) and progression free survival (PFS). However, it is important to consider the pharmacokinetics, pharmacodynamics, personal preferences, economical concerns and side effect profile of individual drugs while using as a single agent or in combination to avoid further compromising the affected organ system of a susceptible patient. Published literature and guidelines highlight the benefits or disadvantages of particular regimens in patients with specific risk factors such as National Comprehensive Cancer Network, International Myeloma Working group, European Myeloma Working group and Mayo Stratification for Myeloma and Risk-Adapted guidelines. The purpose of this review is to identify and summarize findings from original studies and guidelines for personalized therapy in themanagement of MM.
Methods and Results:
A comprehensive literature search was carried out using PubMed, EMBASE, Wiley Cochrane library, Scopus, Web of Science, CINAHL, and Clinicaltrials.gov (updated 7-29-2017) for original articles, institutional, national and international guidelines addressing the adjustments of myeloma therapy with a focus on personalized therapy. 129 articles focusing on individualization of therapy for MM patients with renal impairment, risk of venous thromboembolism, high-risk cytogenetics, bone disease, advance age, relapse and plasma cell leukemia are selected and summarized. Articles and guidelines regarding personalization of stem cell transplantation and therapy options after relapse are also summarized in this review.
Conclusion:
First step towards personalizing the MM therapy is identification of comorbidities (DM, HTN), organ function status (heart, renal failure), disease risk stratification (standard, intermediate, high), transplant eligibility (yes/no), and other risk factors (advanced age, marrow suppression, neuropathy, fluid overload, hyperglycemia). For elderly frail patients, the dosage of all drugs needs to be adjusted considering the age-related compromise of organ systems. Transplant eligibility needs to be considered before using melphalan based induction regimens. Maintenance therapy with novel agents increases OS and PFS but their long term use is associated with drug specific adverse effects. Lenalidomide is associated with bone marrow suppression and second primary malignancies (Kotchetkov et al., 2017). Thalidomide is associated with neuropathy worsening (Mohty, et al., 2010) and emergence of treatment resistant clones. Bortezomib is associated with neuropathy, herpes viral reactivation but is considered superior approach especially for high relapse risk population. In advance renal impairment (RI) patients, thalidomide and lenalidomide can cause hyperkalemia and pancytopenia respectively therefore, may require dose adjustment. Bortezomib is preferred in advanced RI and shows rapid improvement in kidney function. Melphalan and cyclophosphamide dose should be reduced in patients with RI (Dimopoulos et al., 2010). High dose steroids and immune modulators are associated with venous thromboembolism (De Stefano V et al. 2014) therefore, these drugs should be used with risk appropriate (low risk prophylaxis with ASA, high risk with full anticoagulation) prophylaxis. MM patients with high-risk cytogenetics should receive risk adapted bortezomib or carfilzomib based three drug induction therapy and long-term maintenance therapy preferably with a proteasome inhibitor. Bisphosphonate dose in MM patients with RI should be reduced and alternative options should be explored (Denosumab) if necessary.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal